Indications
- Bone defect due to trauma, infection, tumor resection, etc.

DOH-MD-No. 003103
衛部器廣字第11210011號
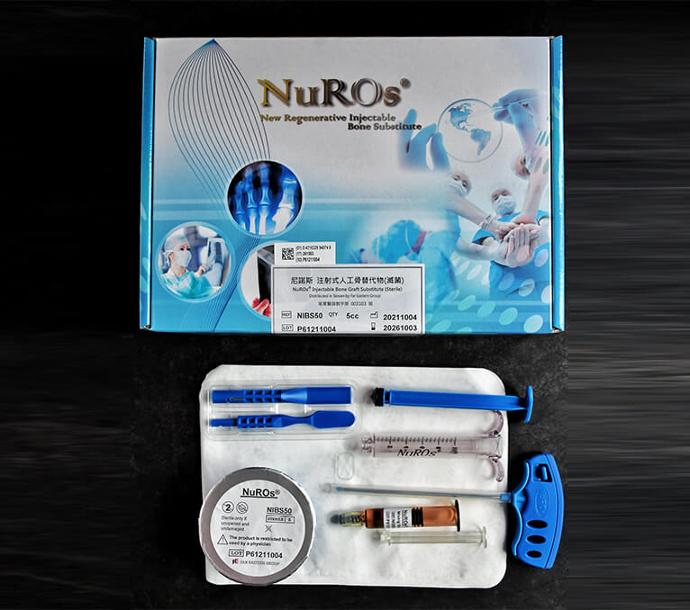
Specifications And Contents
Full version
The product include powder, liquid solution, stir stick, trocar, and dispenser
| Specifications And Contents |
Specifications And Contents |
| NIBS15 | 1.5 c.c. |
| NIBS30 | 3 c.c. |
| NIBS50 | 5 c.c. |
| NIBS60 | 6 c.c. |
| NIBS100 | 10 c.c. |
Simple version
The products include powder, jar, and stir stick
| Specifications And Contents |
Specifications And Contents | Saline solution |
| NIBS15 | 1.5 c.c. | 0.75~1.0 ml |
| NIBS30 | 3 c.c. | 1.5 ~ 2.0 ml |
| NIBS50 | 5 c.c. | 2.5 ~ 3.0 ml |
| NIBS60 | 6 c.c. | 3.0 ~ 4.0 ml |
| NIBS100 | 10 c.c. | 5.0 ~ 6.0 ml |
| NBS200 | 20 c.c. | 10.0 ~ 12.0 ml |
Adverse Effects And Complications
Successful results may not be achieved in every surgical case. If any complication occurs,additional surgery to remove or replace the implant may be required. Complications include but are not limited to :
- Implant fraction, migration.
- Loss of contour.
- Tissue thinning over implant site.
- Edema, redness or infection.
Contraindications
Each surgeon must evaluate prudently before operation, if there is any contraindication. Contraindications may include but are not limited to :
- Use for stress bearing applications
- Use in a currently infected field or surgical site near an infection
- Use for stress bearing applications
- Use in a currently infected field or surgical site near an infection
- Use in the implant site that can’t provide structural support to bone cement
- Use in patients who have not reached an age at which bones and skeleton growth is essentially complete
- Use in patients with any metabolic bone disease
- Pregnancy and breastfeeding feminine
- Defects due to disease or congenital malformation
- Use in patients with immunologic abnormalities and systemic disorders which result in poor wound healing or will result in tissue deterioration over the implant site.
Warranty And Precautions
- NuROs® is for single use. Resterilize or use it if the package is opened or damaged will cause cross inflection.
- The curative effect of NuROsR will be reduced by moist air, so NuROsR must be applied immediately after being opened.
- After mixing the powder and the solution, it must be applied to the surgical site in ten minutes and must avoid touching the implant site hardly during setting time.
- Mix the powder with the mixing solution completely before use. The effect of preparing NuROs with any other substance, including antibiotics and blood, is unknown.
- When severe bleeding occurs, stop bleeding before applying. Excess body fluid may affect the curative effect.
- NuROs® is used for bone repair only, and every step of treatment will be followed on aseptic handling technique.
- Store at cool dry place for extending expiration date.
- If there is any product damage or you have any questions, contact your sales or customer service via +886-3-599-7135.